Surface Tension
- It has been seen that a drop of blood forms a hump on horizontal glass. Similarly a drop of mercury forms a near perfect sphere and can be rolled just like a steel ball over a smooth surface.
- In these observations, liquid droplets behave like small balloons filled with the liquid and surface of the liquid acts like a stretched elastic membrane under tension. These pulling forces that causes this tension acts parallel to the surface and is due to attractive forces between the molecules of the liquid. The magnitude of this force per unit length is called surface tension (σ) and is expressed in unit N/m.
- To visualize that how surface tension arise at interface, consider the microscopic view of molecule on surface
and inside the liquid filled in a container. The attractive force applied on the interior molecule by the surrounding molecules balance each other because of symmetry. But for the molecule on the surface or at interface between two different mediums, the attractive forces are not symmetric. The attractive forces applied
by the air/gas molecule above are usually small. Therefore, there is a net attractive force acting on the molecule at the surface of the liquid. These are balanced by repulsive forces from the molecules below the surface that are trying to be compressed. Due to this, liquid minimise its surface area. This is the reason for the tendency of liquid droplets to attain a spherical shape which has the minimum surface area for a given volume.
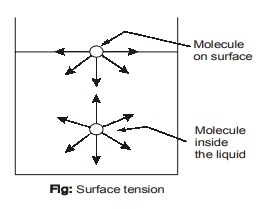
- Surface tension is due to “cohesion” between the liquid particles.
- Whenever a liquid is in contact with other liquids or gases, or solid surface, an interface develops that acts like a stretched elastic membrane, creating surface tension.
- There are two features to this stretched elastic membrane : the contact angle θ, and the magnitude of the
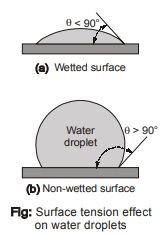 surface tension, σ (N/m). Both of these, depend on both the type of liquid and the type of solid surface ( or other liquid or gas) with which it shares an interface.
surface tension, σ (N/m). Both of these, depend on both the type of liquid and the type of solid surface ( or other liquid or gas) with which it shares an interface.
For example, the car’s surface will get wetted when water is applied to the surface. If before applying water, waxing is done to the car’s surface and then water is applied, the car’s surface will not get wet. This is because of the change of the contact angle from being smaller than 90°, to larger than 90°. The waxing has changed the
nature of the solid surface. - For liquids, surface tension decreases with increase in temperature.
- Due to surface tension, pressure change occurs across a curved interface.
- Surface tension is also defined as work done per unit increase in surface area. This work done is stored in the form of surface energy.
σ = Work done/Area - A liquid droplet takes spherical shape because surface area is minimum in spherical condition. Therefore, the surface energy is minimum. Minimum surface energy leads to most stable state.
<< Previous | Next >>
Must Read: What is Fluid Mechanics?
WhatsApp Group
Join Now
Telegram Group
Join Now

