Clay Minerals
STRUCTURE OF CLAY MINERALS
Clay minerals are composed of two basic structural units.
(i) Tetrahedral unit
(ii) Octahedral unit
(i) Tetrahedral Unit: The tetrahedral unit comprises of a central silicon atom surrounded by four oxygen atoms positioned at the vertices of the tetrahedron.
The tetrahedral units are combined with each other such that each oxygen atom at the base of tetrahedron lies in same plane and is being shared between two tetrahedron units. This combination of tetrahedral units is called silica sheet.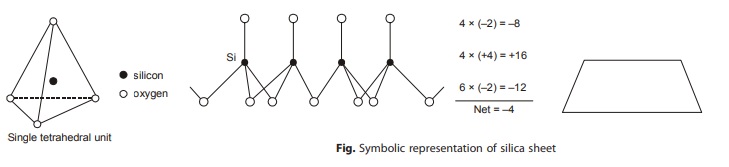
In above silica sheet, each of the oxygen ion at the base is common to two adjacent units. The sharing of charges leaves three negative charges at the base per tetrahedral unit. This, alongwith the two negative charges at the apex, makes a total of five negative charges to four positive charges of silicon ion. Thus, there is a net charge of –1 per unit.
(ii) Octahedral unit: The octahedral unit comprises a central ion of either aluminium, magnesium or iron
surrounded by six hydroxyl ions.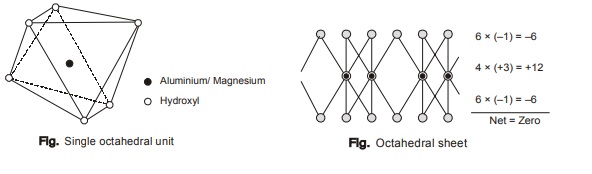
Each hydroxyl ion in Gibbsite sheet is being shared between 3 octahedral units. Therefore, net charge present over Gibbsite is –1.
TYPES OF CLAY MINERALS
Clayey soils are made of three basic minerals:
(i) Kaolinite mineral
(ii) Montmorillonite mineral
(iii) Illite mineral
(i) Kaolinite Minerals:
Kaolinite minerals are made of an aluminium sheet (gibbsite sheet, Gi) combined with a silica sheet (Si). The structural units join together by hydrogen bond, which develops between the oxygen of Silica sheet and the hydroxyls of Alumina sheet. The resulting layer is about 7A° thick. Since hydrogen bond is strongest bond, Kaolinite mineral is quite stable. Thus soils consisting kaolinite mineral do not show much change in volume due to moisture variation. The most common example of kaolinite mineral is china clay. The surface area of the Kaolinite particles per unit mass is about 15 m2/g. The surface area per unit mass is defined as specific surface. It is commonly found in sedimentary and residual soil. There is little or no isomorphous substitution in kaolinite. surface area of the Kaolinite particles per unit mass is about 15 m2/g. The surface area per unit mass is defined as specific surface. It is commonly found in sedimentary and residual soil. There is little or no isomorphous substitution in kaolinite.
(ii) Montmorillonite Minerals:
Montmorillonite mineral is a three layer sheet mineral. Basic three layer sheet units are formed by keeping one silica sheet (Si) on the top and bottom of gibbsite sheet (Gi). Isomorphous substitution of magnesium or iron for the aluminium in the gibbsite sheet is common. It attracts water to form a water layer between two connecting basic units.
The bonding between basic units is by water forces and exchangeable ion linkage. The bonding of these sheets is very weak, and water may enter between the units. Thus mineral has significant affinity for water, and results in expansion and swelling. Bentonite and Black cotton soils contains mont-morillonite mineral in large proportion.
Specific surface of montmorillionite is about 200-800 m2/g.
(iii) Illite Minerals:
Illite is also a three-layer sheet mineral. It consists of basic three layer sheet unit similar to montmorillonite. There is also a substantial amount of isomorphous substitution of silicon by aluminium in silica sheet. Consequently, the mineral has a larger negative charge than that in montmorillonite. The basic units are bonded by non exchangeable potassium (K+) ions, which is stronger than the bond in montmorillonite. Thus illite swells less than montmorillonite. However swelling is more than in kaolinite.
The specific surface is 50-100 m2/g.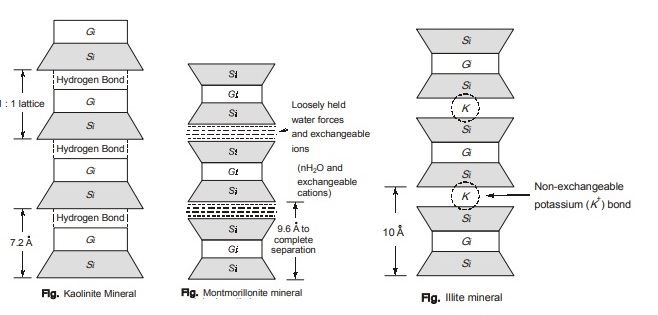
TYPES OF SOIL STRUCTURES
Single Grained Structure
In granular soils, the ratio of volume to surface area is large, so that mass derived (i.e. gravity) forces are dominant and surface-derived electric forces are negligible. These are found in soils having size greater than 0.02 mm. Single-grained structures are formed when the soil grains settle out independently due to gravity force. Examples – gravel and coarse sand
Honeycomb Structures
This type of structures are found in soil having size between 0.02 mm to 0.002 mm. Gravity and surface electric (adhesive) forces, both are responsible for their formation. These structures enclose large volume of voids. When structure is unbroken, these soils have ability to bear large loads, but once the structure is broken, load carrying capacity is lost and show large deformation. Examples – fine sand and silts.
Flocculated and Dispersed Structures
These are found in the soils having size less than 0.002 mm. The structure of a fine-grained cohesive soil can be described fully with the understanding of inter particle forces and the geometrical arrangement of particles.
The fine particles are of flaky or plate like shaped. They have large surface area and therefore surface electric force become dominant. The clay particles have a negative charge on the surface and positive charge on edges. Particles joined edge-to-edge or edge-to-surface results in a flocculated structure. This soils structure have high volume of voids.
Dispersed structures develops in clays that have been remoulded. When flocculated structural soils are remoulded by nature or man, converts its edge-to-edge or edge-to-surface orientation into surface-to-surface orientation, this results in a dispersed structure.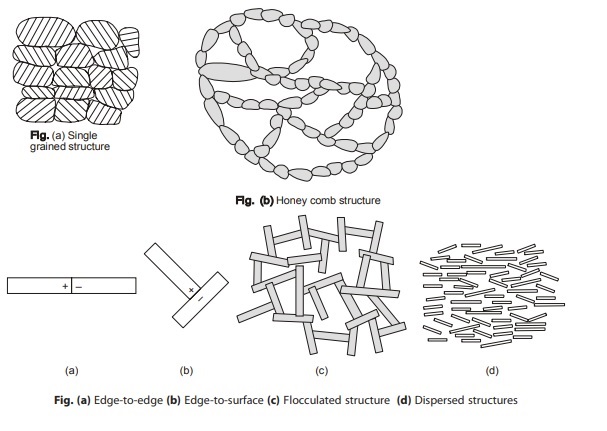
Structure of a Composite Soil
Composite soils are mixture of coarse grain and fine soils.
(a) Coarse Grained Skeleton: Coarse grained skeleton is a composite structure which is formed when soil contain both coarse grain and fine grain particles. Coarse grain particles form a frame work or skeleton. The space between this skeleton is filled by fine grain particles called binders. These types of soils are less compressible.
(b) Clay-matrix Structure: Clay-matrix Structure is also a composite structure which is formed when soil contain both fine grain and coarse grain soils. However, in this case the amount of fine particles is very large as compared to coarse particles. The clay forms a matrix in which coarse grain appear floating without touching each other. Such soils are relatively more compressible.
Compaction
- Almost an instantaneous phenomenon.
- Soil is always partially saturated.
- Densification is due to a reduction in the volume of air voids at a given water content.
- Specified compaction techniques are used in this process.
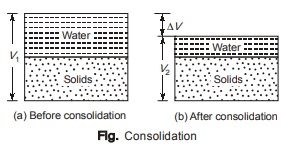
Consolidation
- It is a time dependent phenomenon.
- Soil is completely saturated.
- Volume reduction is due to expulsion of pore water from voids.
- Consolidation occurs on account of a load placed on the soil.