Corrosion in Pipes
When water flows through a metal pipe (such as a cast iron or a steel pipe), it attacks and disintegrates the surface of the pipe. The material of the pipe thus gets dissolved and rusted, thereby reducing the life and carrying capacity of the pipe. This phenomenon which leads to the disintegrating of the pipe is known as corrosion. The corrosion in pipes reduces their life and carrying capacities, and may also impart colour and odour to the flowing water.
There are many reasons of corrosion, but corrosion of metal pipes may often occur due to electrolysis. Stray (D.C) electric currents from any grounded device may enter the pipe, and flow through it to some point of departure. Also when two dissimilar metals are immersed in an electrolyte such as water, an electric potential is developed and an electric current starts flowing between the metals. The metal which lies high in the “electrochemical series” will get dissolved and deposition will start, on the metal which lies low in the “electrochemical series”.
Thus the metal which is high in the series becomes the anode, and the metal which is lower in the series becomes the cathode. This type of potential may develop between the pipe metal and the pipe fittings (of different metal); and also between the pipe metal and other metals present as impurities in the pipe metal. Thus, if the material of the pipe fittings are such as to fall in the electrochemical series at a place lower than that of the pipe metal then, itself naturally electrolysis will set up and the pipe metal will go on dissolving. The dissolved metal being washed down by the flowing water, and thus making it impure. The corrosion continues indefinitely, if not checked, thus leading to the complete destruction of the pipe.
Factors Responsible for Corrosion
- Oxygen content of the water
- pH valve
- Temperature and Soil bacteria
- Moisture content
- Composition of pipe material
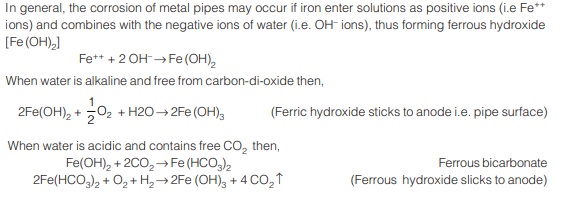
This ferric hydroxide [Fe(OH)3] is in the form of insoluble red precipitate and gets deposited on the pipe surface. Further, this leads to the formation of tubercules of ferriec hydroxide on the inside surface of the pipe, this process is known as “tuberculation”. Tuberculation leads to increase in pipe roughness and hence reduce the carrying capacity.
Corrosion Control
Corrosion of metal pipes may be reduced in various ways as described below:
Protective Coating
- Pipe surfaces are coated with coatings of paint, galvanizing bituminous compounds, cement lining. etc.
- Red lead paint and zinc pigment are used for painting the exteriors of the pipes.
Selecting proper pipe material
- The pipe metal may be chosen to be more resistant to corrosion
- Certain alloys of iron or steel with chromium, copper or nickel found to be better than the pure iron and steel.
- If steel or iron are to be used then they should be as pure as possible.
Quality of water
- The water passing through the pipe should be made as less corrosive as possible.
- This can be accomplished by raising the pH of water by adding alkalinity in the form of lime or powdered chalk.
- By reducing the dissolved O2 and CO2.
- By adding chemical compound which reduces tuberculation such as sodium hexa-metaphosphate.
Cathodic protection
- Electrolytic corrosion can be prevented by connecting the pipe with negative terminal of a D.C. generator and positive terminal with blocks of zinc or magnesium buried in the ground near the pipe.
TESTING OF THE PIPE LINES
After the pipe lines has been laid, fitted with all appurtenances and accessories, painted both from inside as well as outside by means or protective paints etc., the pipe line will be tested for the soundness in its construction.
Steps to be followed for testing of the pipes are as:
- The pipe line is tested section by section. Thus, at a time only one particular section lying between two sluice valves is taken up for testing.
- The downstream sluice valve is closed and water is admitted into the pipe through the upstream sluice valve, the air valve will be properly operated during filling up of the pipe.
- The upstream valve, through which water was admitted, is closed so as to completely isolate the pipe section from the rest of the pipe.
- Pressure gauges are then fitted along the length of the pipe section at suitable intervals on the crown
- The pipe section is then connected to the delivery side of a pump through a small bypass valve, and the pump is started so as to develop pressure in the pipe.
- The by-pass valve is then closed and the pumping is disconnected.
- The pipe is thus kept under pressure for 24 hours and inspected for possible defects, leakage at the joints. This completes the test.
- The pipe is emptied, and the observed defects are rectified so as to make the line fit for use.
Chemical Characteristic
Chemical characteristics are result of the solvent properties of water and they are often important in specifying waste water quality. Important chemical characteristics of wastewater are listed below:
(a) Total solids, suspended solids and settleable solids
(b) pH value
(c) Chloride content
(d) Nitrogen content
(e) Presence of fats, greases and oils
(f) Sulphides, sulphates and H2S gas
(g) Dissolved oxygen
(h) Chemical oxygen demand (COD)
(i) Theoretical oxygen demand (ThOD)
(j) Total organic carbon
(k) Bio-chemical oxygen demand (BOD)
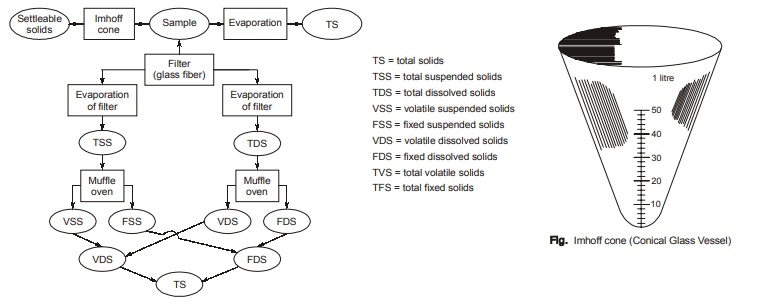
Total Solids, Suspended Solids and Settleable Solids
Solids present in sewage may be in any of the four forms, suspended solids, dissolved solids, colloidal solids and settleable solids. Suspended solids are those solids which remain floating in sewage. Dissolved solids are those which remain dissolved in waste water just as salt in water. Colloidal solids are finely divided solids remaining either in solution or in suspension. Settleable solids are that portion of solid matter which settles out, if the waste water is allowed to remain undisturbed for a period of 2 hours.
• Inorganic matter consist of sand, gravel, debris, chlorides, sulphates etc.
• Organic matter consist of
– Carbohydrates such as cellulose, cotton, fibre, sugar etc.
– Fats and oils from kitchen, garages, shops etc.
– Nitrogenous compounds like proteins, urea, fatty acids etc.
It is to be noted that in general, presence of inorganic solids in waste water is not harmful and they can be removed by mechanical appliances. However, organic solids whether suspended or dissolved can cause nuisance if they are disposed off without treatment. The amount of various solids present in waste water can be determined as follows:
The amounts of various kinds of solids present in waste water can be determined as follows:
- The difference between the total solids (S1) and the suspended solids (S2) represent dissolved solids plus colloidals or filterable solids (S3)
i.e. S3 = S1 – S2.
- As we know that total suspended solids(S2) can be either volatile or fixed. If their proportion is to be determined, then the suspended solids (S2) in step (b) is burnt and ignited at about 550°C in an electro furnace for about 15 to 20 minutes. Loss of weight will represent the amount of volatile solids in volume of sample filtered through filter. Let, the volatile suspended solid concentration by S4(in mg/l).
- Now, fixed suspended solid concentration (S5) can be calculated as, S5 = S2 – S4.
- The quantity of settleable solids (S6) can be determined with the help of a specially designed conical glass vessel known as Imhoff cone as shown in figure. In this cone, sewage is allowed to stand for about 2 hours and quantity of solids settled in bottom of cone represents the quantity of settleable solids.
For better understanding of complete process, flow-chart shown in figure below can be referred:
Dissolved Oxygen (D.O)
Dissolved oxygen present in sewage is very important for respiration of aerobic micro-organism as well as for all other aerobic life forms. Quantity of D.O. indicates the freshness of sewage. The dissolved oxygen in fresh sewage depends upon temperature. If the temperature of sewage is more, the D.O. content will be less.
Maximum quantity of D.O. that can remain mixed in water at a particular temperature is called Saturation Dissolved Oxygen. The D.O. content of sewage is generally determined by the Winkler’s Method which can be described as follows:
- Collect a water sample in a clean glass bottle and add 1 ml of MnSO4 solution per litre of sample. This helps to oxidise any organic matter in the sample, which can interface with the oxygen measurement.
- Add 1 ml of alkaline iodide – azide reagent per litre of sample and mix thoroughly. This reacts with any dissolved oxygen in the sample, converting it to iodide ions.
- Add 1 ml of concentrated H2SO4 per litre of sample, taking care to avoid splashing. This reacts with the iodide ions to produce iodine.
- Allow the solution to stand for at least 10 minutes to allow the iodine to fully develop. The solution turns yellow-brown.
- Add 1 ml of starch solution per litre of sample, and mix throughly. This will cause the solution to turn blue-black.
- Titrate the solution with adding Na2S2O3 solution drop by drop until the blue-black colour disappears. This indicates that all of the iodine has been reacted with the thiosulphate.
- Volume of thiosulphate used represents the amount of oxygen that was originally present in the water sample.
Laboratory estimation of kD and L0 by Thomas’s graphical method
Value of kD can be computed from BOD values measured at various time duration (in days). The sewage samples are tested for BOD at different time durations. A graph is now plotted between value of function
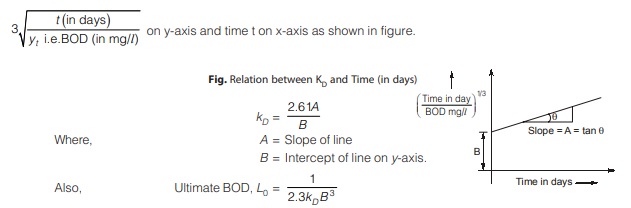
<< Previous | Next >>
Must Read: What is Environmental Engineering?
Dear Aspirants,
Your preparation for GATE, ESE, PSUs, and AE/JE is now smarter than ever — thanks to the MADE EASY YouTube channel.
This is not just a channel, but a complete strategy for success, where you get toppers strategies, PYQ–GTQ discussions, current affairs updates, and important job-related information, all delivered by the country’s best teachers and industry experts.
If you also want to stay one step ahead in the race to success, subscribe to MADE EASY on YouTube and stay connected with us on social media.
MADE EASY — where preparation happens with confidence.

MADE EASY is a well-organized institute, complete in all aspects, and provides quality guidance for both written and personality tests. MADE EASY has produced top-ranked students in ESE, GATE, and various public sector exams. The publishing team regularly writes exam-related blogs based on conversations with the faculty, helping students prepare effectively for their exams.

