Chlorination
Disinfecting Action of Chlorine
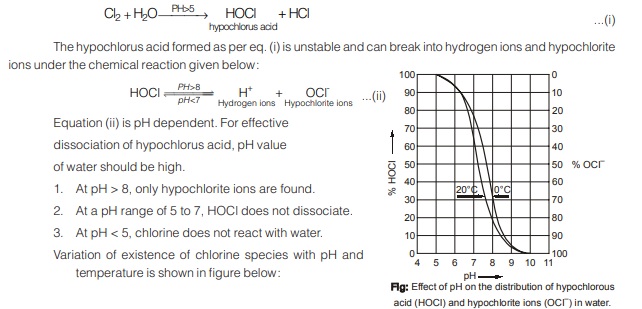
Addition of chlorine to water forms hypochlorous acid or hypochlorite ions which have an immediate and disastrous effect on most of microscopic microorganisms. The reaction that takes place are:
Doses of Chlorine
Inorganic and organic impurities present in water affects the amount of chlorine that is to be used in water. When chlorine is added to water, firstly, it reacts with all the inorganic impurities such as reducing agents like S2–, Fe2+, Mn2+, NO2 – etc. These agents convert chlorine into chloride which has no residual oxidising power. Chlorine added after this point is consumed by ammonia to form chloramines. Simultaneously, it reacts with organic impurities present in water sample. Some organic impurities completely oxidise chlorine while some chloro-organic compounds formed will have some oxidising power. Reaction of chlorine with organics for combined chlorine residuals are usually insignificant in comparison to chloramines. The chlorine consumed in all the above reactions represents chlorine demand of water.
A large chlorine residual in water may cause bad tastes, whereas, a small residual may not be relied upon. Disinfection of water is generally satisfactory if free residual chlorine is about 0.2 mg/l after 10 minutes of contact period. This optimum dose of chlorine for a given water is, determined by adding varying amounts of chlorine to a given sample and observing the residual left after a contact period of about 10 minutes. The dose leaving a residual of about 0.2 mg/l is selected as optimum dose for that water.
TYPES OF CHLORINATION
Break-Point Chlorination:
Break point chlorination is a term which gives us an idea of the extent of chlorine added to water. Infact, it represents that much dose of chlorination, beyond which any further additional chlorine will appear as free residual chlorine.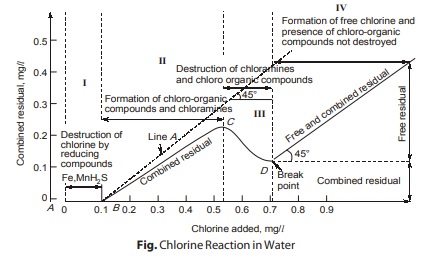
- As raw water has a chlorine demand therefore curve A is generally does not obtained between residual chlorine and applied chlorine. During the disinfection process, the amount of residual chlorine will be very less in beginning (marked by stage I) in figure, during which various chemicals such as ions of ferrous, sulphides or nitrites present in water, will be oxidised.
- Stage-II reflects the formation of combined residuals as the ammonia or amines reacts with HOCl so chloramines that has formed. During state II, as the demand for disinfection is satisfied, combined residual chlorine goes on increasing till a point C is reached where amount of combined residual is maximum. The stage of point C is accompanied of bad taste and odour indicating the starting of stage III.
- During state-III, oxidation of organic matter present in water starts. Therefore, after point C, with increase in applied chlorine, residual chlorine goes on decreasing shown by curve CD in figure in This stage, free chlorine breaks down chloramines into nitrogen compounds. At point D, bad smell and taste suddenly disappear showing that oxidation of organic matter is completed. At point D, residual chlorine has minimum value.
- Further addition of applied chlorine results in increase in residual chlorine as represented by line E, the slope of which is 45° as shown in figure during stage IV.
Orthotolidine Test
The main test used for residual chlorine for many years has been that using orthotolidine. One of the reasons of it is that, orthotolidine is not recognized as being among those chemical substances capable of causing cancer.
In this test, 10 ml of chlorinated water sample is taken in a test tube after the required contact period. To this, 0.1 ml of orthotolidine solution is added.
The colour formed is noted after 5 minutes. The presence of yellow colour indicates the presence of chlorine (free or combined) in water. By comparing this colour with a colour of known concentration, the amount of chlorine residue is obtained. If free and combined chlorine are to be found out separately, then colour formed in 5 seconds (R1) and colour formed in 5 minutes is noted. R1 will correspond to free chlorine and R2 will correspond to free and combined chlorine both.
In orthotolidine test, presence of iron, manganese, nitrite etc. will give false yellow colour thereby indicating increased chlorine residue which is wrong. For such water, orthotolidine arsenite test is performed. In this case, sodium arsenite is added to chlorinated water. This will dechlorinate the sample.
To this dechlorinated sample, orthotolidine solution is added and colour formed R1 is noted. Now R1 will correspond to quantity iron, manganese and nitrite. After that, another sample of chlorinated water is taken and colour formed in 5 seconds (R2) and colour formed in 5 minutes (R3) is noted. Now, R2 will correspond to combination of free chlorine and interfering ions such as iron , manganese and nitrite while R3 will correspond to combination of free and combined chlorine and interfering ions such as iron , manganese and nitrite.
Relative effectiveness of components of free available chlorine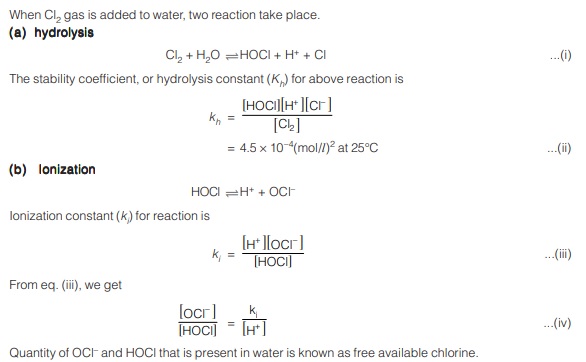
Removal of Temporary Hardness
As discussed in earlier chapters, temporary or carbonate hardness is caused by carbonates and bicarbonates of calcium and magnesium. It can be removed by adding lime or by simply boiling the water as explained below:

<< Previous | Next >>
Must Read: What is Environmental Engineering?

