Cement and Lime
Following points of differences may be noted between ordinary cement and lime:
- The cement is used for the gain of early strength whereas lime gains the strength slowly.
- The color of cement and lime are different.
- The cement and lime both acts as binding material having good ultimate strength but lime experiences less early strength as compare to cement.
- The cement is having good heat of hydration due to which it sets early as compared to other binding material like lime.
MANUFACTURING OF CEMENT
- The cement is manufactured by integrating the calcareous component and argillaceous component in ratio of 3 : 1.
- The calcareous component can be limestone, chalk, marine shells, marl whereas, argillaceous components can be shale, clay, blast furnace slag, slate.
- The calcareous component is used to derive the ingredient called lime whereas the argillaceous component composed of silica, alumina, iron oxide and other impurities.
- The manufacturing of cement can be done through the following two processes i.e. dry and wet processes.
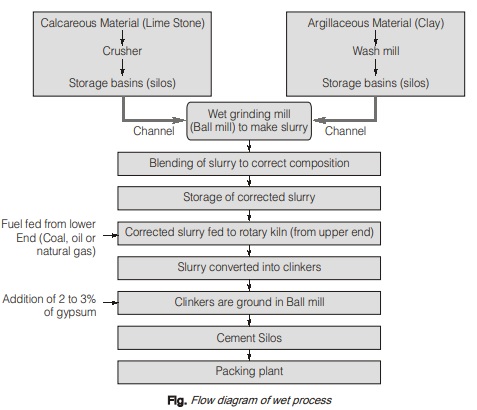
(a) Wet process:
- It is the oldest method of manufacturing cement which is now-a-days obsoleted.
- It is a costly method because it requires higher degree of fuel consumption, power consumption etc.
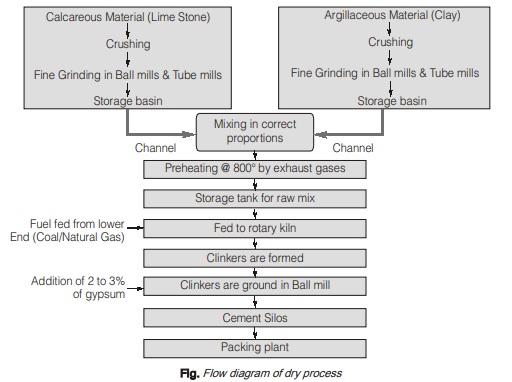
(b) Dry process:
- It is a new method of manufacturing cement which is trending now-a-days. The fuel consumption and power consumption has been reduced to a greater extent by modifying the wet process.
Composition of Cement Clinker (Bougue’s compound)
- The major compounds that are formed when cement reacts chemically with water are shown in table.
Principal Compounds in Portland Cement
| The principal mineral compounds in Portland cement | Formula | Name | Symbol | Percentage |
|---|---|---|---|---|
|
1. Tricalcium silicate 2. Dicalcium silicate 3. Tricalcium aluminate 4. Tetracalcium alumino ferrite |
3CaO . SiO2
2CaO . SiO2 3CaO . Al2O3 4CaO . Al2O3 . Fe2O3 |
Alite
Belite Celite Felite |
C3S
C2S C3A C4AF |
30-50% 20-45% 8-12% 6-10% |
- Besides major compounds, minor compounds such as Soda (Na2O) and Potash (K2O) are also formed. These two minor compounds are chiefly responsible for Efflorescence in cement concrete and cement mortar.
- It is found that ordinary cement achieves 70 percent of its final strength in 28 days and that of 90 percent in 1 year cent in 1 year.
- The strength in a cement is majorly depends upon the Bougue’s compound. The properties of Portland cement varies significantly with the proportion of four Bougue’s compounds.
Constituents of Cement
- The relative proportions of these oxide compositions are responsible for influencing the various properties of cement.
- Consequently, free lime will exist in the clinker and will result in an unsound cement. An increase in silica content at the expense of alumina and ferric oxide makes the cement difficult to fuse and form
clinker. - Rate of setting of cement paste is controlled by regulating the ratio SiO2/(Al2O3 + Fe2O3).
- When development of heat of hydration is undesirable, the silica content is increased to about 21 percent. and the alumina and iron oxide contents are limited to 6 per cent each.
- Resistance to the action of sulphate waters is increased by raising further the silica content to 24 percent and reducing the alumina and iron contents in 4 percent each.
- Small percentage of iron oxide renders the highly siliceous raw materials easier to burn.
Table: Contituents of Cement
| Constituents | Percentage | Average percentage |
|---|---|---|
|
Lime (CaO) Silica (SiO )2 Anumina (Al O )2 3 Calcium Sulphate (CaSO )4 Iron oxide (Fe O )2 3 Magnesia (MgO) Sulphur Soda and Potash (Na O + K O)2 2 |
62 to 67%
17 to 25% 3 to 8% 3 to 4% 3 to 4% 0.1 to 3% 1 to 3% 0.5 to 1.3% |
62 22 5 4 3 2 1 1 |
- Lime (CaO): This is the important ingredient of cement and its proportion is to be carefully maintained. The lime in excess makes the cement unsound and causes the cement to expand and disintegrate. On the other hand, if lime is in deficiency, the strength of cement is decreased and it causes cement to set quickly.
- Silica (SiO2): This is also an important ingredient of cement and it imparts strength to the cement due to the formation of dicalcium and tricalcium silicates. If silica is present in excess quantity, the strength of cement increases but at the same time, its setting time gets prolonged.
- Alumina (Al Alumina (Al2O3): This ingredient imparts quick setting property to the cement. It acts as a flux and lowers the clinkering temperature. However, high temperature is essential for the formation of a suitable type of cement and hence, the alumina should not be present in excess amount as it weakens the cement.
- Calcium Sulphate (CaSO4): This ingredient is added in the form of gypsum and its function is to increase the initial setting time of cement.
- Iron Oxide (Fe2O3): This ingredient imparts colour, hardness and strength to the cement.
- Magnesia (MgO): This ingredient, if present in small amount, imparts hardness and colour to the cement. A high content of magnesia makes the cement unsound.
- Sulphur (S): A Sulphur very small amount of sulphur is useful in making sound cement. If it is in excess, it causes unsoundness in cement.
- Alkalies: The most of the alkalies present in raw materials are carried away by the fuel gases during heating and the cement contains only a small amount of alkalies. If they are in excess in cement, they cause a number of troubles such as alkali-aggregate reaction, efflorescence and staining when used in concrete, brickwork or masonry mortar.
<< Previous | Next >>
Must Read: What is Construction Material?
WhatsApp Group
Join Now
Telegram Group
Join Now

