Anaerobic Stabilisation Units
Design Data of Septic Tank
Flow of sewage is taken as 40 to 70 litres/capita/day. When sludge is also discharged into septic tank, flow is taken as 90 to 150 litres/capita/day. Rate of accumulation of sludge = 30 litres/person/year. A free board of about 0.3 m may be provided above the top sewage line in the tank. This will help accommodate the scum in the tank. The inlet and outlet baffles or tees should extend up to top level of the scum (about 22 cm above the top sewage line), but must stop a little below the bottom of the covering slab (by at atleast 7.5 cm or so), so as to allow for the free movement of gases. The inlet should penetrate by about 30 cm below the top sewage line and the outlet should penetrate to about 40% of the depth of sewage. Further, the outlet invert level should be kept 5 to 7.5 cm below the inlet invert level. Detention period for septic tank generally varies between 12 to 36 hours, but is commonly adopted as 24 hours. Cleaning period are generally varies from 6-12 months. Septic tanks are usually rectangular with their length about 2 to 3 times of width. The width should not be less than 90 cm. The depth of the tank generally ranges between 1.2 to 1.8 cm. Volume of septic tank = (sewage flow × detention time) + (sludge accumulation rate × cleaning period). The tank effluent coming out from the septic tank is no better than the effluent of an ordinary sedimentation tank. It contains larger amount of putrescible organic matter (200 to 250 mg/l)
Effects of Air Pollution on Human Health
- The adverse effect of air pollution on human health has remained the prime consideration in air pollution.
- Sulphur Dioxide (SO2) : SO2 is an irritant gas which effects mucous membrane when inhaled. It leads to bronchial spasms. Asthma patients are badly affected.
- Carbon Monoxide (CO) : Carbon Monoxide has a strong affinity for combining with the haemoglobin of the blood to form carboxyhaemoglobin, COHb. This reduce the ability of the haemoglobin to carry oxygen to the body tissues. CO has about two hundred times the affinity of oxygen for attaching itself to the haemoglobin so that low levels of CO can still result in high levels of COHb. Carbon Monoxide also affects the central nervous system.
- Oxides of Nitrogen : Among several oxides of nitrogen known to exist in the ambient air, only two are thought to affect human health. These are nitric oxide (NO) and nitrogen dioxide (NO2). It causes eye and nasal irritation and pulmonary discomfort.
- Hydrogen sulphide and Mercaptans : H2S is a foul smelling gas. It is well known for its rotten egg like odour. Exposures to hydrogen sulphide for short periods can result in fatigue of the sense of smell.
- Ozone : It is a gas that has an irritant action in the respiratory track, reaching much deeper into lungs than the oxides of sulphur.
- Fluorides : Since fluoride is cumulative poison, hydrogen fluoride is less harmful to human beings.
- Lead : The major source of lead in urban atmosphere is the automobile. The effects include gastrointestinal damage of liver and kidney damage, abnormalities in fertility and pregnancy and mental development of children gets affected.
- Hydrocarbon Vapours : The effect of it is primarily irritating. It is a major contributor to eye and respiratory irritation caused by photochemical smog.
- Carcinogenic Agents : These are responsible for cancer. For example, the poly cyclic organic compound, 3, 4-benzpyrene. The origin of these compounds is in the incomplete combustion of hydrocarbons.
- Insecticides : These are not only harmful for insects but also poisonous for human like DDT (Dichloro Diphenyl Trichloroethylene). They can affect the central nervous system and may attack other vital organs. Insecticides/pesticides can also causes premature labour and abortion, due to high concentration of presticides in the body of expectant mothers.
- Radioactive Isotopes : The main radioactive isotopes that may reach ambient air and Iodine 131, Phosphorous 32, Cobalt 60, strontium 90, Radium 226, Carbon 14, Sulphur 35, Calcium 45 and Uranium. The serious health effects are anaemia, leukaemia and cancer radioactive isotopes also cause genetic defects and sterility as well as embryo defects and congenital malformation.
- Allergic Agents : Allergic Agents are generally recognised by medical personnel that the air we breathe is the natural carrier of many microscopic organic materials which may act as allergens. Our body reactions to such allergens occur mainly in the skin and the respiratory tract.
Effects of Air Pollution on Plants
- The adverse impacts of air pollution are not limited to human health alone but plants are also affected by air pollutants.
- The most prominent air pollutant, which cause severe damage to the plants is fluorine, emitted from factories manufacturing aluminium, glass, phosphate, fertilizers etc. Its concentration in excess of about 0.3 µg/m3 cause photo-toxicological effects on plants.
- The damage caused by the air pollutants like SO2, H2F, HCl, Cl2, O3, NOx, NH3, Hg, H2S, PAN, herbicides, smog etc., to the plants and vegetation occurs in the leaf structure. The pollutants clog the stomata of the leaf, thereby reducing the intake of CO2, which adversely affects the photosynthesis.
- Dry Cement-kiln dust appears to cause little damage deposited on a leaf surface, yet in the presence of moisture, such dust imparts damage and consequential growth inhibition to plant tissues.
OZONE LAYER DEPLETION
- Ozone layer depletion is the most dreaded aspect of air pollutions, having wide spread implication, extending over the entire atmosphere. This problem is caused by the reduction of naturally available ozone layer in the atmosphere.
- Out of all earlier described zones of the atmosphere, the second zone i.e. the stratosphere, remains the most important to man, as it is the stratosphere which primarily contains ozone gas (O3) chiefly in the layers between 25 and 40 km above the ground level.
- This ozone layer cut off short wave length radiations (called ultraviolet radiation) from reaching the surface of the earth. Therefore, this process serves as a protective shield to human life against the adverse effects of UV like burn and skin cancer. It is obvious that any depletion of stratosphere ozone would be harmful to life on this earth. Hence ozone layer is termed as ozone umbrella.
- Primary reason for ozone layer depletion is CFC (chlorofluorocarbon) or freons. CFC contains chlorine, fluorine and carbon and it does not occur by itself in nature, but is produced only due to human activities. The freons are a group of chlorofluorocarbons used as aerosol propellants, refrigerants, solvents and as gases for the production of foamed plastics.
- Ozone is destroyed due to the photolytic reaction of CFC as shown below.
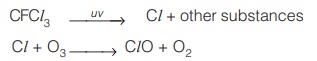
- Methane, destroys Cl and thus affords protection to the ozone layer. Similarly, NO2 reacts with Cl, and helps to prevent the depletion of ozone layer.
- When there is no chlorine present in fluorocarbons, they are called hydrofluorocarbons. These substances are a very important replacement for chlorofluorocarbon because they pose no threat to the ozone layer as they do not contain chlorine.
- Due to presence of ozone layer, the UV rays do not reach the surface of the earth, and the temperature does not rise. The carbon dioxide in the atmosphere does not allow the release of the reflected solar radiation striking the earth’s surface. Thus it protects the heat from being lost out of the global set up.
- Thus ozone and carbon dioxide both control the temperature on the earth.
<< Previous | Next >>
Must Read: What is Environmental Engineering?
Dear Aspirants,
Your preparation for GATE, ESE, PSUs, and AE/JE is now smarter than ever — thanks to the MADE EASY YouTube channel.
This is not just a channel, but a complete strategy for success, where you get toppers strategies, PYQ–GTQ discussions, current affairs updates, and important job-related information, all delivered by the country’s best teachers and industry experts.
If you also want to stay one step ahead in the race to success, subscribe to MADE EASY on YouTube and stay connected with us on social media.
MADE EASY — where preparation happens with confidence.

MADE EASY is a well-organized institute, complete in all aspects, and provides quality guidance for both written and personality tests. MADE EASY has produced top-ranked students in ESE, GATE, and various public sector exams. The publishing team regularly writes exam-related blogs based on conversations with the faculty, helping students prepare effectively for their exams.

